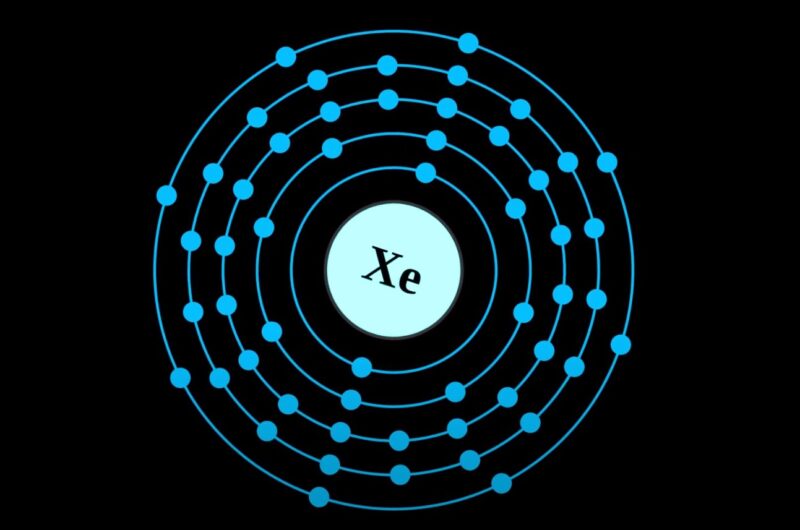
Xenon (Xe), a noble gas in Group 18, is stable and non-reactive due to its full valence shell. Valence electrons are crucial for chemical bonding, as they determine an atom’s ability to form molecules.
Xenon is unique among noble gases; it can form compounds under special conditions, defying the belief that noble gases are inert. This makes Xenon’s valence electrons a key area of study in chemistry, revealing insights into atomic behavior and bonding.
Determining the Valence Electrons
In the field of chemistry, identifying the number of valence electrons in an element is fundamental to understanding its chemical properties and bonding behavior. This section focuses on determining the valence electrons of Xenon, a noble gas in Group 18 of the periodic table.
The process of finding the Valence Electrons
To determine the number of valence electrons in Xenon, one must first understand its position in the periodic table. Xenon is located in Group 18, which indicates that it has eight valence electrons.
These electrons are found in the outermost shell of the atom, which is the primary factor in an element’s chemical reactivity and bonding behavior.
Electron Configuration: [Kr]4d¹⁰5s²5p⁶
Xenon’s electron configuration is represented as [Kr]4d¹⁰5s²5p⁶. This notation indicates that Xenon has the same electron configuration as Krypton (Kr) up to the 4d orbital, which is filled with ten electrons.
The 5s and 5p orbitals contain two and six electrons, respectively, summing up to a total of eight valence electrons in the outermost shell. These eight electrons fulfill the octet rule, contributing to Xenon’s stability and low reactivity.
Valence Electrons Count
Xenon, with its atomic number of 54, has a total of eight valence electrons. These electrons are located in the atom’s outermost shell and are responsible for the chemical properties of the element.
Having eight valence electrons makes Xenon very stable, as it has achieved a complete octet, which is the most energetically favorable arrangement for an atom. This full valence shell is the reason why Xenon, along with other noble gases, is generally unreactive and rarely forms compounds.
The presence of eight valence electrons is significant because it means that Xenon does not readily gain or lose electrons, making it an exception to the typical chemical reactivity patterns of other elements.
Reactivity and Formation of Compounds

Xenon is a noble gas that is generally unreactive due to its complete outer electron shell. However, it can form compounds under certain conditions. The formation of xenon compounds typically involves highly electronegative elements, such as fluorine or oxygen.
These compounds are formed when the valence electrons of xenon are attracted by the electronegative elements, allowing for chemical bonding.
Examples of Compounds
Xenon forms several stable compounds with fluorine, including XeF₂ (xenon difluoride), XeF₄ (xenon tetrafluoride), and XeF₆ (xenon hexafluoride). Xenon difluoride is a colorless solid that is used as a strong oxidizing agent.
Xenon tetrafluoride is a white crystalline substance, and xenon hexafluoride is a powerful fluorinating agent. These compounds exhibit different properties and are used in various chemical applications.
Deeper Explanation
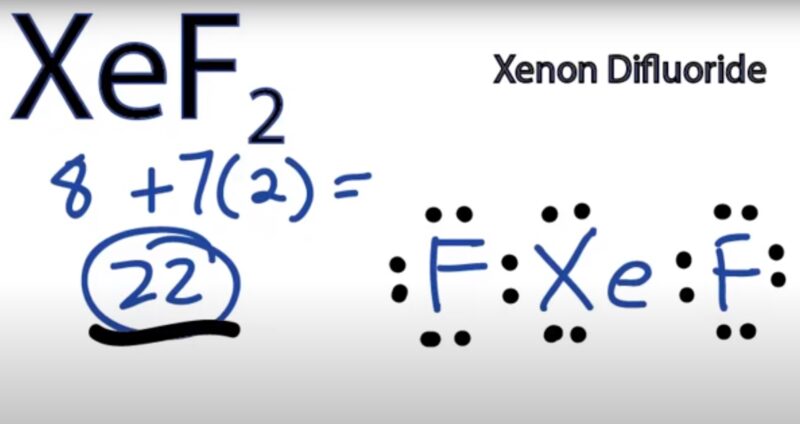
Xenon can form compounds under special conditions that provide enough energy to overcome its natural inertness. These conditions include high pressure, high temperature, and the presence of highly electronegative elements like fluorine and oxygen.
For example, xenon difluoride (XeF₂) is prepared by heating an excess of xenon with fluorine at 673K and 1 bar pressure in a sealed nickel tube.
“Full Octet”
Xenon achieves a “full octet” because it has eight valence electrons in its outermost shell, satisfying the octet rule. The octet rule states that atoms are most stable when they have eight electrons in their valence shell, which is the case for xenon.
Its electronic configuration is [Kr]4d¹⁰5s²5p⁶, indicating a complete outer shell.
Properties and Applications of Compounds
Xenon compounds, such as XeF₂, XeF₄, and XeF₆, have unique properties and applications. XeF₂ is a colorless solid used as a strong oxidizing agent.
XeF₄ is a white crystalline substance, while XeF₆ is a powerful fluorinating agent used in various chemical applications. Xenon is also used in medical imaging and as a general anesthetic due to its non-toxic and non-reactive nature.
Ongoing Research
Recent research is expanding our understanding of xenon, traditionally known for its inertness, revealing its potential in various fields. The discovery that xenon can form compounds has revolutionized our view of noble gases.
In medicine, xenon is recognized for its safe anesthetic properties and is being explored for its neuroprotective effects and use in lung imaging. In materials science, xenon’s inert nature is being harnessed to develop novel, stable materials.
While its direct role in environmental remediation is still emerging, xenon’s chemistry could inspire new pollution control methods. These advancements suggest a future where xenon’s unique properties are integral in healthcare, environmental solutions, and material innovation.
FAQ
Why is it considered unique among noble gases?
Xenon is unique because, unlike most noble gases, it can form compounds under special conditions like high pressure and the presence of highly electronegative elements. This ability challenges the traditional view of noble gases as completely inert.
Can it form stable compounds?
Yes, Xenon can form several stable compounds, especially with fluorine, such as XeF₂, XeF₄, and XeF₆. These compounds are stable and have various chemical applications, defying the earlier belief that noble gases cannot form stable compounds.
What role do Xenon’s valence electrons play in its chemical behavior?
Xenon’s eight valence electrons, which fill its outermost shell, make it generally unreactive and stable. However, under certain conditions, these electrons can participate in chemical bonding, allowing Xenon to form compounds.
Is it used in any practical applications?
Yes, beyond its chemical interest, Xenon has practical uses. It’s used in medical imaging and as a general anesthetic due to its non-toxic and non-reactive nature. Its compounds, like XeF₂, are used as oxidizing agents in chemical processes.
How does it defy the octet rule?
While Xenon follows the octet rule by having eight valence electrons, it defies the rule in the sense that it can form compounds despite having a full valence shell. This is unusual for noble gases, which are typically inert due to their complete octet.
What ongoing research involves this gas?
Current research is exploring Xenon’s potential in various fields. In medicine, it’s being studied for its neuroprotective effects and use in lung imaging. In materials science, its inert nature is being utilized to develop novel, stable materials. Additionally, its unique chemistry could inspire new methods of pollution control.
Final Words
Xenon, a noble gas in Group 18, stands out for its full valence shell, granting it stability and low reactivity. This gas, traditionally viewed as inert, can form compounds under specific conditions, challenging our understanding of noble gases.
Its eight valence electrons, located in the outermost shell, are key to its chemical behavior, particularly in forming compounds like XeF₂, XeF₄, and XeF₆ with fluorine. These discoveries in xenon chemistry are not just academic; they have practical implications in fields like medicine, where xenon is used for its anesthetic properties, and materials science, where its inert nature is exploited for creating stable materials.
Xenon’s unique properties and ongoing research into its capabilities highlight its potential in advancing healthcare, environmental sustainability, and material innovation. To look deeper into the world of chemistry, feel free to explore our article on the aniline compound next.