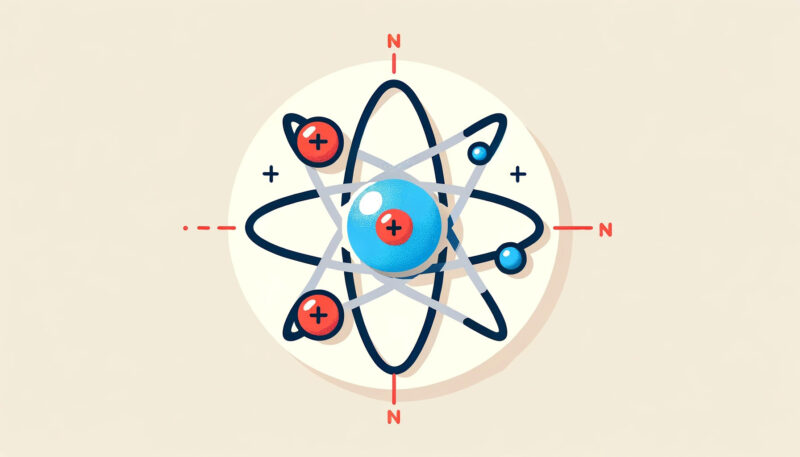
At the core of each atom lies a nucleus, a densely packed center, where two types of particles reside: protons and neutrons. Protons carry a positive charge, contributing to the atom’s identity and behavior in chemical reactions.
Neutrons, though chargeless, play a crucial role in stabilizing the nucleus. Surrounding this nucleus in a cloud of rapid movement are electrons, minuscule particles with a negative charge.
These three components, each with distinct characteristics, collaborate to give an atom its unique properties, including its chemical reactivity and physical state.
Key Takeaways
- Subatomic particles, including protons, neutrons, and electrons, are the foundational elements of matter, influencing everything from chemical properties to technological advancements.
- Exploration of these particles has led to significant breakthroughs in medicine, energy, and material science, revealing the profound impact of atomic research on our daily lives and the universe at large.
Protons, Neutrons, and Electrons Explained
Atoms, the building blocks of matter, are composed of three primary particles: protons, neutrons, and electrons. Each of these particles plays a pivotal role in defining the characteristics and behavior of atoms.
Protons
Positively charged particles are located in the atom’s nucleus. The number of protons in the nucleus defines the atomic number, which is unique for each element on the periodic table.
This number not only identifies the element but also determines its chemical properties and place in the periodic table. The presence of protons imparts an overall positive charge to the nucleus, which is balanced by the negatively charged electrons.
Neutrons
The second type of particle found in the nucleus, is neutral, having no charge. They are approximately the same size as protons but are slightly heavier. Neutrons play a crucial role in the stability of the nucleus.
In elements with a higher atomic number, neutrons prevent the protons from repelling each other due to their like charges. The number of neutrons can vary within atoms of the same element, leading to different isotopes. Isotopes have the same number of protons but different numbers of neutrons, which affects their atomic mass but not their chemical properties.
Electrons
Third type of particle, with a negative charge. They are much smaller than protons and neutrons and orbit the nucleus in various energy levels or shells.
The arrangement of electrons in these shells determines how an atom will interact and bond with other atoms. It is these interactions and bonds that form molecules and define the chemical properties of matter.
Electrons are also responsible for electrical conductivity and play a vital role in many physical phenomena.
| Particle | Charge | Location | Relative Mass | Interesting Fact |
|---|---|---|---|---|
| Proton | +1 | Nucleus | 1 (1.6726 x 10-27 kg) | Protons determine the element’s identity and are used in proton therapy to treat cancer. |
| Neutron | 0 (Neutral) | Nucleus | 1 (1.675 x 10-27 kg) | Neutrons are the key to the stability of the nucleus and are responsible for the phenomenon of nuclear fission. |
| Electron | -1 | Orbiting the Nucleus | 1/1836 (9.109 x 10-31 kg) | Electrons can behave both as particles and waves, a fundamental concept in quantum mechanics. |
How Are These Particles Related?
Beyond the fundamental trio of protons, neutrons, and electrons, lies a diverse array of particles and forces that govern the universe at its most fundamental level.
Quantum Quirks and Quarks
The quantum world, where these particles exist, operates under rules drastically different from our everyday experiences. One of the most intriguing aspects is the dual nature of particles like electrons.
They exhibit both particle-like and wave-like behavior, a phenomenon known as wave-particle duality. This concept is at the heart of quantum mechanics and challenges our traditional understanding of physics.
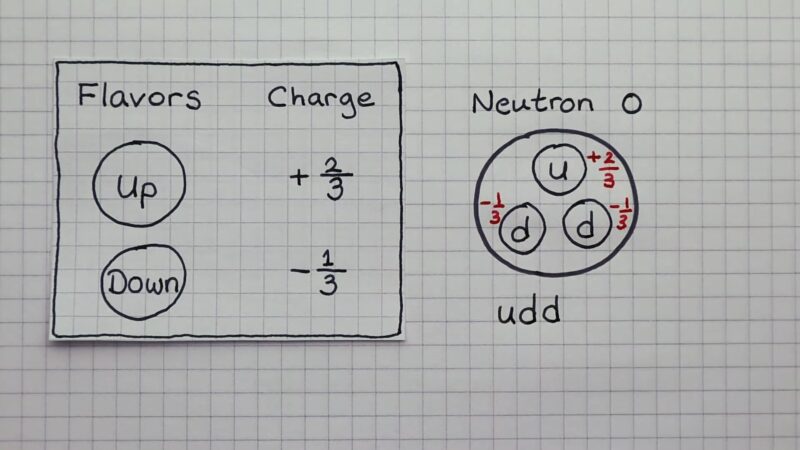
Quarks are another fundamental component, even smaller than protons and neutrons. These particles come in six types, or ‘flavors’ – up, down, charm, strange, top, and bottom.
Protons and neutrons are each made up of three quarks. It is the combination of different quarks and their properties, like ‘color charge’, that determines the type and nature of the larger particles they form.
The Forces That Bind
Four fundamental forces govern the interactions between these particles. The strong nuclear force, the strongest of the four, is responsible for holding protons and neutrons together in the nucleus.
This force is mediated by particles called gluons, which ‘glue’ quarks together. Then there’s the electromagnetic force, which causes the attraction and repulsion between charged particles.
The weak nuclear force is responsible for radioactive decay and neutrino interactions. Lastly, gravity, the weakest yet most far-reaching force, acts between all particles with mass.
Antimatter and the Universe’s Secrets
For every particle, there exists an antiparticle with the same mass but opposite charge. When matter and antimatter meet, they annihilate each other, releasing energy.
The existence of antimatter and the mystery of why the universe consists primarily of matter, with very little antimatter, is one of the most intriguing questions in physics.
Neutrinos: The Ghostly Particles
Neutrinos are among the most abundant particles in the universe but are notoriously difficult to detect because they rarely interact with matter. These ‘ghost particles’ are incredibly light and can pass through entire planets without being affected.
Studying neutrinos can provide valuable insights into processes like nuclear fusion in stars and supernova explosions.
Applications in Everyday Life
The study of subatomic particles has led to practical applications that significantly impact our daily lives. For instance, the World Wide Web was initially developed at CERN (European Organization for Nuclear Research) to facilitate information sharing among scientists working on particle physics.
PET scans, a crucial medical imaging technology, are based on the detection of positrons, the antimatter counterpart of electrons.
The Large Hadron Collider
The Large Hadron Collider (LHC) at CERN is the world’s largest and most powerful particle accelerator. It has been instrumental in significant discoveries, including the Higgs boson, a particle that gives mass to other particles.
The ongoing experiments at LHC and other facilities worldwide continue to push the boundaries of our understanding, seeking answers to fundamental questions about the nature of the universe and the very fabric of reality.
The world’s largest and most powerful particle accelerator, once experienced an unusual interruption in 2009 due to a bird. A baguette dropped by a bird onto outdoor machinery led to a temporary power loss and a significant delay in operations.
Technological and Scientific Advances
The exploration of subatomic particles has not only expanded our understanding of the universe but also fueled remarkable technological and scientific advancements. These breakthroughs, rooted in the manipulation and comprehension of atomic and subatomic structures, have led to innovations in various fields, ranging from medicine to energy production.
Nuclear and Particle Therapy
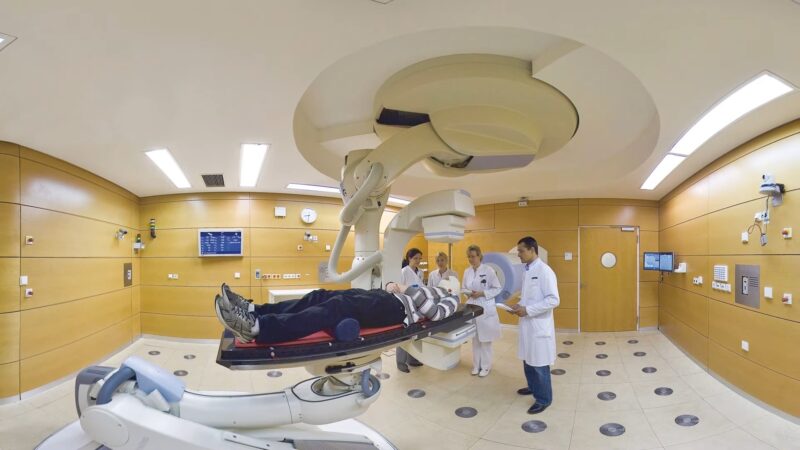
In the medical field, the understanding of atomic particles has led to revolutionary treatment methods. Radiotherapy, for instance, uses high-energy radiation, often in the form of gamma rays or X-rays, to target cancer cells.
This treatment exploits the interaction of radiation with matter at the atomic level to destroy cancerous cells while sparing surrounding healthy tissue. Particle therapy, a more advanced form, uses protons or heavy ions such as carbon.
These particles can be controlled with greater precision, focusing the maximum energy directly on the tumor with minimal impact on surrounding tissues. This precision reduces side effects and improves treatment outcomes, especially in sensitive areas like the brain.
Nuclear Energy: A Powerful and Controversial Resource
The manipulation of atomic nuclei has given rise to nuclear energy, a powerful source of electricity. Nuclear reactors generate energy through fission, where heavy atomic nuclei split into smaller parts, releasing a vast amount of energy.
This process, harnessed in power plants, provides a significant portion of the world’s electricity. However, nuclear energy comes with challenges, including radioactive waste disposal, the potential for accidents, and concerns about nuclear proliferation.
Did you know that the first artificial nuclear reactor, known as the Chicago Pile-1, was constructed under the stands of Stagg Field, a football stadium at the University of Chicago? This groundbreaking experiment, led by physicist Enrico Fermi in 1942, marked the dawn of the nuclear age. It was the first controlled nuclear chain reaction and laid the foundation for the development of nuclear power plants and nuclear weapons. Interestingly, despite its monumental importance, the reactor had no containment structure and was built with minimal safety measures by today’s standards. The successful demonstration of controlled nuclear fission in Chicago Pile-1 was a pivotal moment in science and technology, heralding a new era of energy generation and ushering in complex debates around nuclear energy’s potential and risks.
Advancements in Material Science
The study of subatomic particles has also advanced material science. Understanding the atomic structure and bonding has led to the development of new materials with specific properties.
Superconductors, materials that conduct electricity without resistance at very low temperatures, are a result of this research. These materials have applications in magnetic resonance imaging (MRI), and maglev trains, and could revolutionize energy transmission.
Particle Accelerators in Industry and Research
Particle accelerators, like the Large Hadron Collider, have applications beyond pure scientific research. In industry, accelerators are used for inspecting welds, purifying wastewater, and even in the production of semiconductors.
In research, they help in understanding the properties of materials under high energy, leading to new insights and innovations.
The irradiation process in accelerators is used to strengthen the plastic used in eyeglass lenses, making them more scratch-resistant.
The Quest for Quantum Computing
Quantum computing is another frontier being explored, thanks to our understanding of subatomic particles and quantum mechanics. Quantum computers, based on quantum bits or qubits, have the potential to perform calculations at speeds unimaginable with classical computers.
This technology could revolutionize fields like cryptography, drug discovery, and complex system simulation.
Environmental Monitoring and Protection
At the atomic level, techniques such as isotopic analysis allow for precise environmental monitoring. This includes tracking pollution sources, understanding climate change through ice core analysis, and studying ocean circulation patterns.
These insights are crucial for environmental protection and formulating policies to combat climate change.
FAQs
Can protons exist on their own?
Yes, protons can exist on their own, either in plasmas where the temperature is too high for them to combine with electrons, or in cosmic rays where they travel at high speeds in interstellar space. Protons can also be emitted from some nuclei during radioactive decay.
What is inside a proton?
A proton is not an elementary particle, but a composite particle made of three quarks and gluons. Quarks are the smallest known constituents of matter, and gluons are the particles that carry the strong force that binds the quarks together. The quarks and gluons inside a proton are constantly interacting and changing, creating a complex and dynamic structure.
What is the charge of 0?
The charge of 0 is the net electric charge of an object or a system that has no excess of positive or negative charges. It means that the object or the system is electrically neutral and does not attract or repel other charged objects. An example of an object with a charge of 0 is a neutral atom that has the same number of protons and electrons.
Why can’t we see atoms?
We can’t see atoms with our eyes or with optical microscopes because they are much smaller than the wavelength of visible light. To see an object, it has to reflect or absorb light, but atoms do not interact with light in a way that we can detect. To see atoms, we need to use special instruments that use electron beams or other methods to probe their structure.
What is smaller a Planck or a quark?
A planck is smaller than a quark, because a planck is the smallest possible unit of length in physics, while a quark is a finite-sized particle. The planck length is about 10^-35 meters, which is much smaller than the estimated size of a quark, which is about 10^-18 meters. According to some theories, lengths smaller than the Planck length do not make any physical sense.
The Bottom Line
The study of these particles has led to groundbreaking medical treatments, alternative energy sources, and innovations in material science. Facilities like the Large Hadron Collider are at the forefront of uncovering new scientific truths.
This exploration not only deepens our understanding of the universe but also continues to unlock new potentials for the future, highlighting the significance of atomic and subatomic research.