How Many Valence Electrons Does Xenon (Xe) Have? – Unveiled!
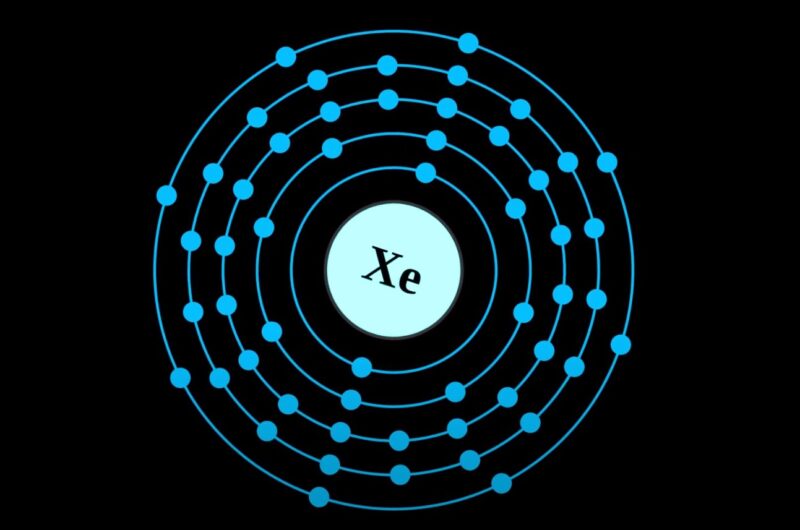
Xenon (Xe), a noble gas in Group 18, is stable and non-reactive due to its full valence shell. Valence electrons are crucial for chemical bonding, as they determine an atom’s ability to form molecules. Xenon is unique among noble gases; it can form compounds under special conditions, defying the belief that noble gases are inert. … Read more