Carbon dioxide, or CO2, is a gas that you’ve probably heard about a lot. It’s what we exhale when we breathe and what plants use during photosynthesis.
But have you ever wondered about its shape at the molecular level? In this post, I will walk you through CO2’s molecular geometry.
Key Takeaways
- CO2 has a linear molecular geometry with a 180-degree bond angle due to symmetrical electron distribution.
- The molecule’s shape is determined by double bonds between carbon and oxygen, with no lone pairs on the carbon atom.
- CO2’s linear geometry and electron sharing are crucial for its roles in the greenhouse effect and photosynthesis.
- Understanding CO2’s structure helps in grasping its environmental impact and the importance of innovative solutions for its management.
What Does CO2 Look Like on a Tiny Scale?
Building Blocks
Imagine CO2 as a simple, straight line with a carbon (C) atom in the middle and an oxygen (O) atom on each end. This arrangement is not random but a result of how these atoms share electrons.
Carbon is like a kid holding hands with two oxygen atoms on either side, forming what we call double bonds. Each pair of hands represents the sharing of four electrons between carbon and oxygen.
The Shape
So, what shape does CO2 take? Picture a straight line; that’s exactly how CO2 looks. It has a linear shape, meaning all the atoms are in a straight line.
This shape is due to the way electrons are spread out around the carbon atom, keeping everything straight and balanced.
Why Is CO2 Shaped That Way?
The Role of Electrons
Electrons are like tiny particles that move around the atoms, and they play a big role in determining the shape of molecules like CO2.
In CO2, the electrons are spread out in such a way that they cancel out any pull in one direction or another, keeping the molecule straight.
Bond Angles
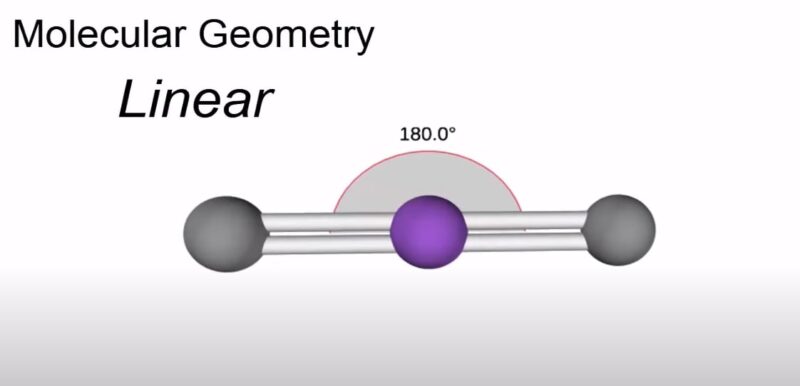
When we talk about the angle between the bonds in a molecule, we’re looking at how wide apart the atoms are spread. In CO2, this angle is 180 degrees, a straight line.
This wide angle is a result of the electrons being shared equally and pushing the oxygen atoms as far apart as possible.
How CO2 Bonds Form
Hybridization
CO2 has something called sp hybridization. This fancy term just means that the carbon atom mixes up its electron orbits a bit to make room for strong bonds with the oxygen atoms.
It’s like rearranging your room to make it more comfortable for your friends to hang out.
The Lewis Structure
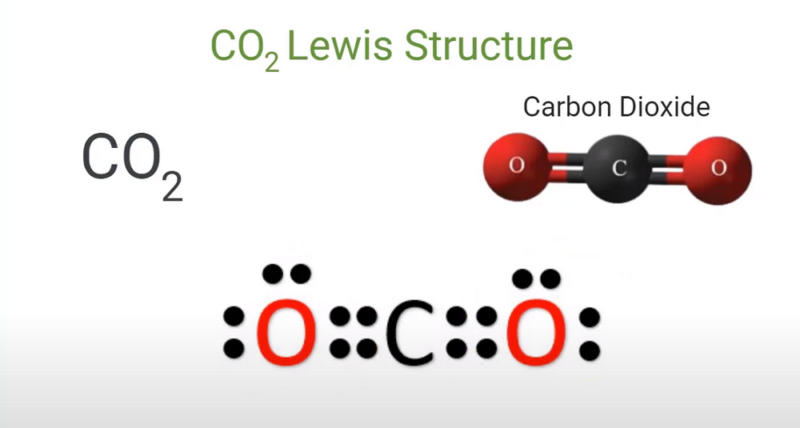
The Lewis structure is a simple way to show how atoms bond and share electrons. For CO2, you draw the carbon atom in the middle and connect it with straight lines (representing double bonds) to the oxygen atoms on each side.
It’s a neat way to visualize how the atoms hold together with no extra electrons hanging around.
Impact Beyond the Shape
While understanding the molecular geometry of CO2 is fascinating, it’s also worth noting the broader implications of this simple molecule.
CO2 plays a significant role in our planet’s climate and ecosystem. Its ability to absorb and emit infrared radiation makes it a key greenhouse gas, influencing Earth’s temperature.
This characteristic, while essential for life by maintaining our planet’s warmth, also contributes to climate change when CO2 levels rise significantly.
The Role of CO2 in Photosynthesis

On a more positive note, CO2’s molecular structure also makes it a key player in photosynthesis, the process by which plants convert light energy into chemical energy.
Plants absorb CO2 from the air, using the carbon to grow and releasing oxygen as a byproduct.
This process not only sustains plant life but also plays a crucial role in maintaining the balance of oxygen and carbon dioxide in the Earth’s atmosphere.
The Balance of Life
The simple linear molecule of CO2 is at the heart of both the challenge of climate change and the fundamental processes that sustain life on Earth.
Its role in the greenhouse effect and photosynthesis highlights the delicate balance of natural processes that our survival depends on.
Knowing CO2’s molecular geometry and its implications helps us appreciate the complexity of our planet and the importance of taking action to protect it.
Carbon Capture and Utilization

One of the most exciting areas of research involves capturing CO2 directly from the air or industrial sources and converting it into useful products.
This technology not only helps reduce the concentration of CO2 in the atmosphere but also provides a sustainable alternative to fossil fuels.
Products like synthetic fuels, plastics, and even concrete can be created using captured CO2, showcasing the molecule’s versatility beyond its role in the greenhouse effect.
FAQs
Why Does CO2 Have a Linear Shape?
CO2’s linear shape comes from the even distribution of electrons, which pushes the oxygen atoms as far apart as possible, resulting in a straight line.
What Is Hybridization in CO2?
Hybridization in CO2 refers to the carbon atom mixing its electron orbits to form strong, stable bonds with the oxygen atoms, leading to a linear shape.
Can CO2 form more than one type of molecular geometry?
No, CO2 consistently forms a linear molecular geometry due to the symmetrical arrangement of electrons around the carbon atom.
Does temperature affect CO2’s molecular geometry?
No, changes in temperature do not affect the linear molecular geometry of CO2.
Can CO2 ever have a triple bond between carbon and oxygen atoms?
No, CO2 always forms with double bonds between the carbon and oxygen atoms, which is crucial for its linear geometry.
Is it possible for CO2 to have lone pairs on the carbon atom?
No, in CO2, the carbon atom does not have lone pairs of electrons because it shares its electrons with oxygen atoms through double bonds.
How does CO2’s molecular geometry contribute to its greenhouse effect?
The linear geometry allows CO2 molecules to vibrate in specific ways when they absorb infrared radiation, contributing to the greenhouse effect.
Are there any molecules similar to CO2 in terms of molecular geometry?
Yes, diatomic molecules like O2 and N2 also have a linear molecular geometry, but they consist of two identical atoms.
Final Words
Carbon dioxide’s structure might seem complex at first, but it’s just about understanding how atoms share electrons and arrange themselves in space.
With its linear shape and 180-degree bond angles, CO2 is a fascinating example of molecular geometry in action.
Whether it’s part of our breath or a plant’s food, CO2’s simple yet intricate design plays a crucial role in life on Earth.

