Knowing the polarity of molecular ions like BrO3- (bromate ion) is not just a chemistry puzzle – it’s a peek into the fascinating world of molecular structures and their interactions.
Today, I will discuss the question: Is BrO3- polar or nonpolar? By breaking down its molecular structure, bond type, and symmetry, I will provide a comprehensive and accessible insight into its polarity.
Molecular Polarity Explained
Molecular polarity is a key concept in chemistry that determines how molecules interact with each other. It arises from the distribution of electrical charges within the molecule. A molecule is considered polar if it has a net dipole moment, meaning there is an uneven distribution of electron density.
- Dipole Moments: These occur when there is a difference in electronegativity between bonded atoms, leading to partial charges within the molecule.
- Electron Distribution: The more electronegative an atom, the more it attracts electrons towards itself, creating regions of negative and positive charges.
- Polar vs Nonpolar: In simple terms, a molecule is polar if it has charged ends; it’s nonpolar if the electron distribution is even.
Factors Affecting Polarity
Several factors influence the polarity of a molecule:
- Electronegativity: Differences in electronegativity between bonded atoms create partial charges.
- Molecular Geometry: The shape of the molecule plays a crucial role. Symmetrical molecules often cancel out dipole moments.
- Bond Type: Ionic bonds usually lead to polar molecules, while nonpolar molecules often have covalent bonds with equal sharing of electrons.
Molecular Symmetry and Polarity
Symmetry in molecular geometry is a critical factor in determining polarity. A symmetrical molecule, even with polar bonds, can be nonpolar overall if the individual bond dipoles cancel each other out. Conversely, asymmetry in a molecule usually leads to a net dipole moment, making it polar.
The Structure of BrO3-
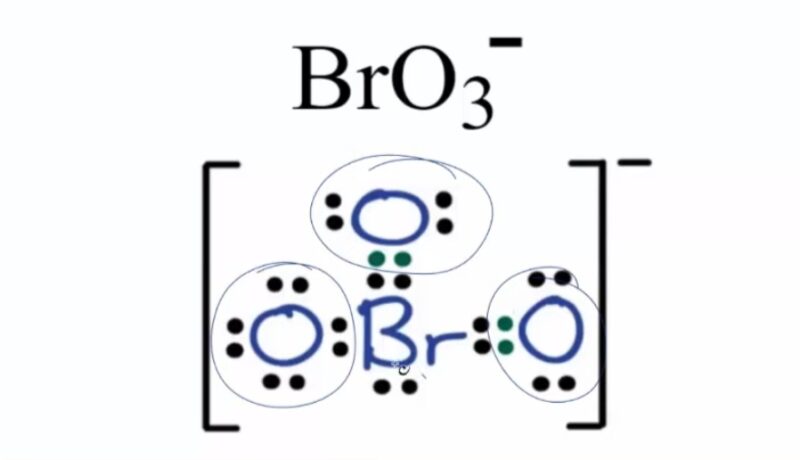
BrO3-, or bromate ion, is composed of one bromine atom and three oxygen atoms. It carries a negative charge, which is distributed over the oxygen atoms. The key to understanding its polarity lies in examining its molecular structure and bond characteristics.
Bonding
In BrO3-, the bromine atom is central, bonded to three oxygen atoms. These bonds are polar covalent, as there is a difference in electronegativity between bromine and oxygen.
- Polarity of Bonds: Oxygen, being more electronegative, pulls the shared electrons closer, creating a partial negative charge on the oxygen atoms and a partial positive charge on the bromine atom.
- Resonance Structures: BrO3- exhibits resonance, meaning its actual structure is a hybrid of multiple Lewis structures. This affects the distribution of the negative charge across the oxygen atoms.
| Molecular Polarity | Effect on Reaction Type | Example |
| Polar | Favorable in ionic and polar covalent reactions | Bromate ion reactions |
| Nonpolar | Favorable in nonpolar reactions | Hydrocarbon reactions |
Geometry
The molecular geometry of BrO3- is trigonal planar. This shape is crucial in assessing its polarity.
- Symmetry in Geometry: The trigonal planar shape is symmetrical. This symmetry could imply that the individual bond dipoles might cancel each other out.
- Effect of Geometry on Polarity: The symmetrical distribution of the oxygen atoms around the bromine atom plays a pivotal role in determining the overall polarity of the molecule.
Polarity Analysis of BrO3-
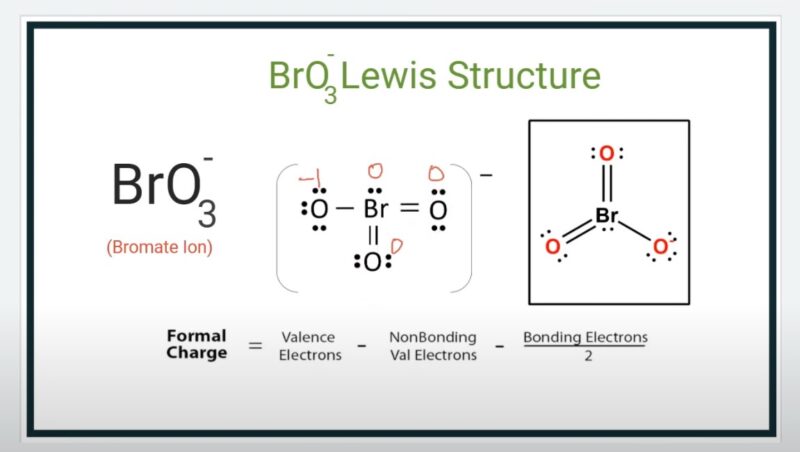
To determine the polarity of BrO3-, one must consider the dipole moment, which is a vector quantity.
- Vector Addition of Dipole Moments: The direction and magnitude of the bond dipoles need to be considered. In a trigonal planar molecule, the bond dipoles can either cancel out or add up, depending on their orientation.
- Net Dipole Moment: The net dipole moment is the resultant of these vector additions. If the net dipole moment is zero, the molecule is nonpolar.
The Role of the Extra Electron
The negative charge on BrO3- adds complexity to the polarity analysis. This extra electron affects the electron density distribution within the molecule.
- Distribution of Negative Charge: The negative charge is delocalized over the oxygen atoms. This delocalization impacts the electron density distribution and, thus, the polarity.
- Influence on Dipole Moment: The extra electron can influence the net dipole moment, either enhancing or diminishing it, based on its distribution.
Practical Applications and Implications

Role in Chemical Reactions
The polarity of BrO3- has significant implications in chemical reactions. As a polar molecule, it interacts differently with various substances:
- Solubility: BrO3- is more soluble in polar solvents like water due to its ability to form dipole-dipole interactions.
- Reactivity: Its polar nature influences its reactivity with other polar or ionic compounds, playing a crucial role in oxidation-reduction reactions.
| Molecular Polarity | Effect on Reaction Type | Example |
| Polar | Favorable in ionic and polar covalent reactions | Bromate ion reactions |
| Nonpolar | Favorable in nonpolar reactions | Hydrocarbon reactions |
Environmental Impact
Having knowledge about the polarity of BrO3- is also crucial from an environmental perspective. Bromate ions can form as byproducts in water disinfection processes and have been identified as potentially harmful.
- Water Treatment: In water treatment, the formation of bromate ions is influenced by factors like pH and the presence of other ions, which are affected by polarity.
- Health Risks: Bromate ions are considered carcinogenic. Understanding their chemical behavior, which is influenced by their polarity, is essential for implementing safer water treatment methods.
Broader Implications in Chemistry
The study of BrO3-‘s polarity is a gateway to understanding broader concepts in chemistry:
- Intermolecular Forces: The polarity of a molecule determines the type of intermolecular forces it can participate in, influencing properties like boiling and melting points.
- Chemical Bonding: A deeper understanding of polar and nonpolar bonds aids in predicting molecular structures and reactivities.
Educational Value
For students and educators, the exploration of BrO3-‘s polarity serves as an excellent educational tool:
- Concept Integration: It integrates various concepts like electronegativity, molecular geometry, and dipole moments.
- Problem-Solving Skills: Analyzing such molecules enhances critical thinking and problem-solving skills, key in scientific education.
The Bottom Line
After examining the bond types, molecular geometry, and electron distribution, it is evident that BrO3- is a polar molecule. Despite its symmetrical shape, the unequal electronegativity between bromine and oxygen, combined with the effect of the extra electron, leads to a net dipole moment.
- Asymmetrical Electron Distribution: The distribution of the negative charge and the differences in electronegativity result in an uneven electron distribution, making BrO3- polar.
- Practical Implications: Understanding the polarity of BrO3- is crucial in predicting its behavior in chemical reactions and interactions with other substances.
FAQs
Can the polarity of BrO3- affect its odor or color?
No, the polarity does not directly affect its odor or color. These properties are influenced by other molecular characteristics.
Does the temperature affect the polarity of BrO3-?
The polarity of BrO3- is a result of its molecular structure and does not change with temperature. However, temperature can affect how it behaves in solutions.
Can BrO3- form hydrogen bonds due to its polarity?
No, BrO3- cannot form hydrogen bonds. Although it is polar, hydrogen bonding requires a hydrogen atom bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine.
Is BrO3- more polar than water?
Comparing polarity directly is complex, but water is considered a highly polar molecule due to its bent structure and significant electronegativity difference. BrO3- is also polar, but its structure is different.
Does the charge on BrO3- contribute to its polarity?
Yes, the negative charge contributes to its polarity by affecting the electron density distribution within the molecule.
Can BrO3- exist as a nonpolar molecule under any conditions?
No, BrO3- cannot exist as a nonpolar molecule under normal conditions since its polarity is inherent to its molecular structure and electronegativity differences between its atoms.
Summary
BrO3-, with its intriguing combination of a symmetrical shape and polar bonds, stands as a polar molecule. This polarity is a result of the electronegativity difference between bromine and oxygen, the geometry of the molecule, and the distribution of the extra electron.
Knowing such molecular characteristics not only enriches our knowledge of chemistry but also aids in the practical application of this knowledge in various scientific fields.